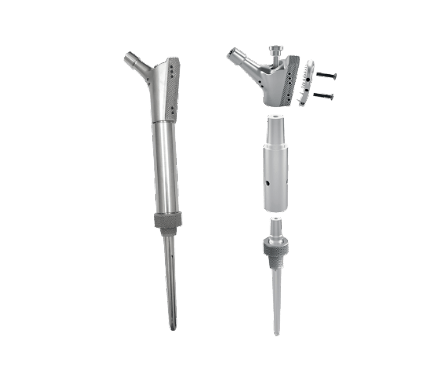
The PowderManufacturing Leader
Adler Ortho® has been a pioneer in the field of 3D printing for Orthopaedic products. Since 2007 we employ that technology to produce standard and custom made implants made either in Titanium or CoCrMo alloy.
 Discover More
Discover More

POWDER MANUFACTURING LEADER
CUSTOM MADESOLUTIONS AND SERVICES
Adler Ortho thanks to its mastery of the 3D printing technique applied to orthopaedics, offers a complete range of services and products entirely designed and custom made.
 Discover More
Discover More
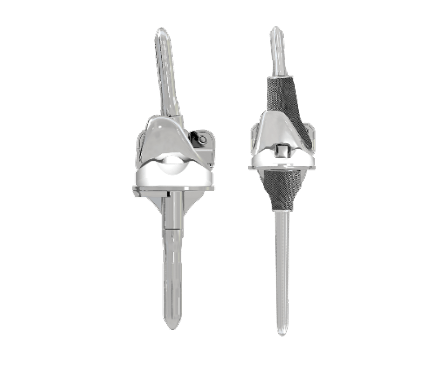
CUSTOM MADE IMPLANTS
AAOS 2024CONGRESS
Adler Ortho® was present from 12 to 16 February 2024 at the American Academy of Orthopaedic Surgeons. This annual meeting offers an experience rooted in education, innovation and exchange for orthopaedic surgery healthcare professionals from around the world.
 Discover More
Discover More

AAOS 2024



POWDER MANUFACTURING LEADER
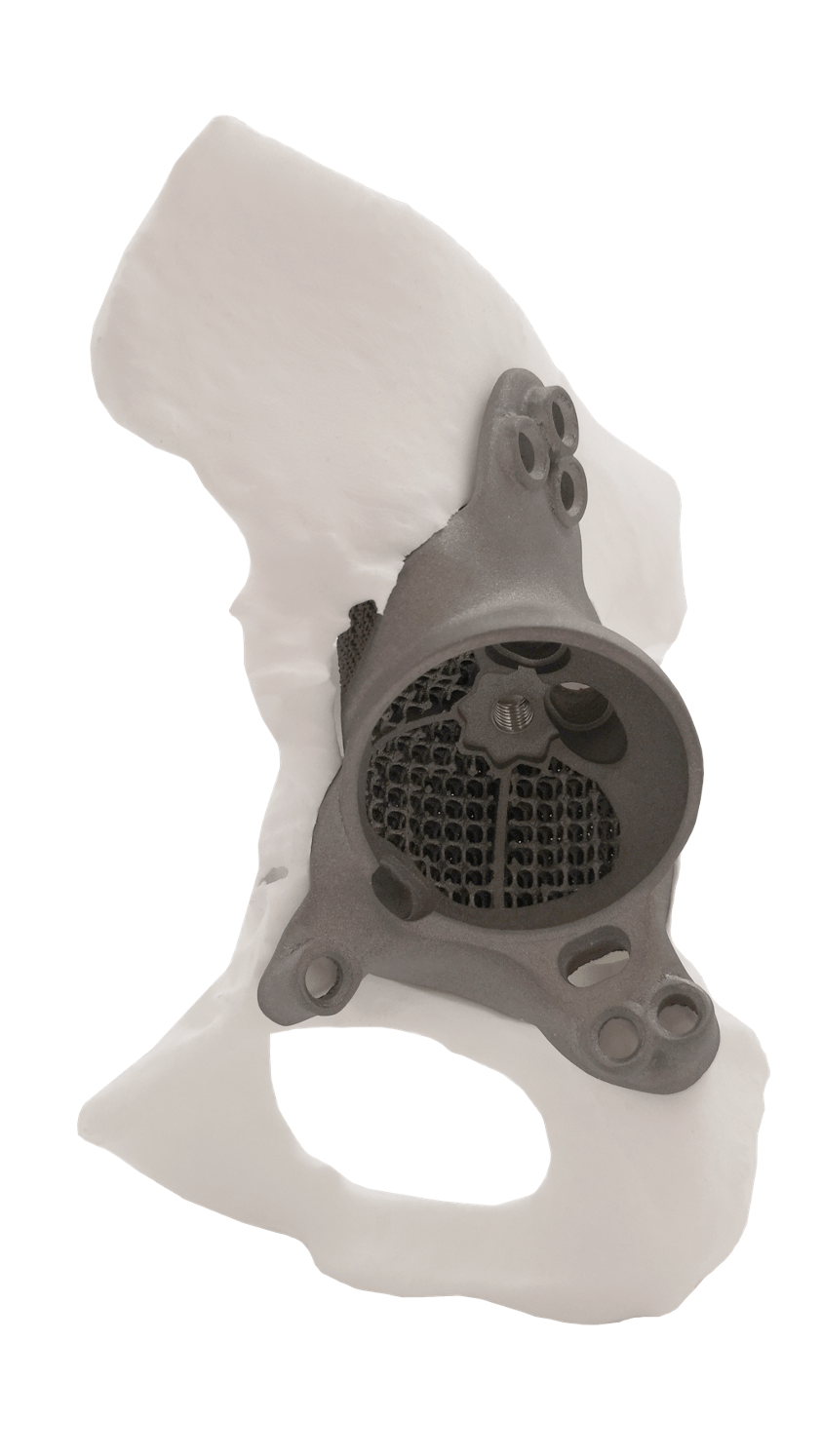
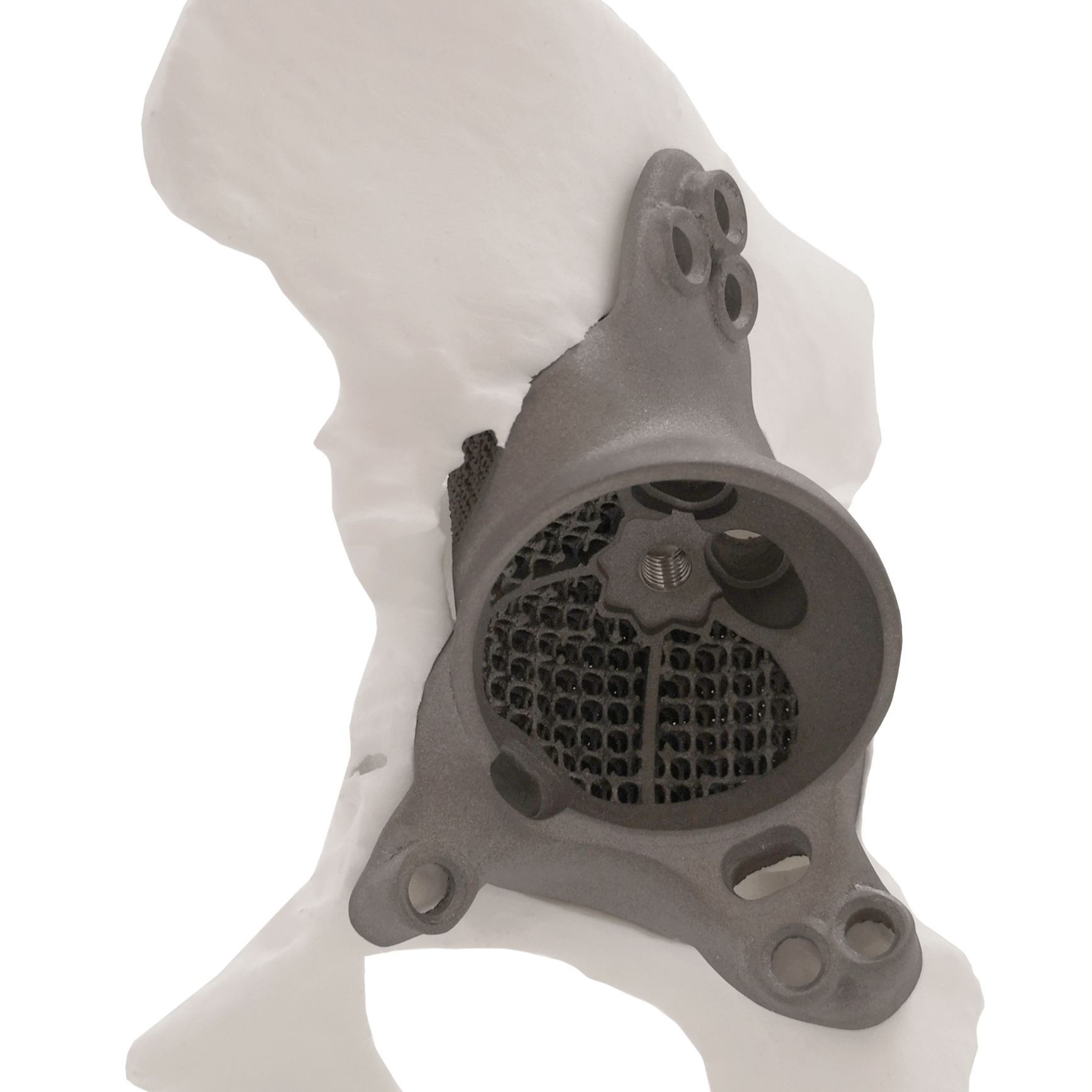
CUSTOM MADE IMPLANTS

AAOS 2024

About US
Adler Ortho® is a privately-owned company fully dedicated to the research, development, production, and sale of medical devices for orthopaedics.
Since its foundation, Adler Ortho® has been driven by innovation, pursuing the employment of the latest technologies available, with vital direction and collaboration from the clinical community.
We are a leading company in the design and marketing of the highest quality orthopaedic implants, mostly produced with 3D printing technology.
Mission
Adler Ortho® is committed to improving patients’ quality of life by developing innovative products employing the latest technologies available, always in strict cooperation with the clinical community.

POWDER MANUFACTURING LEADER
A brief overview of our company and our products
A.C.E
ADLER CENTER
OF EXCELLENCE
Adler Ortho® is actively engaged in a series of medical education activities aimed to maximize the clinical efficacy of its technologies.
OUR POPULAR PRODUCTS
CONTACT US
Adler Ortho® has its headquarters in Cormano, near Milan, Italy. In Italy, we have sales branches in Verona, Bologna, Rome, and Naples. We conduct direct commercial operations in France, the UK, Germany, Austria, Benelux, Switzerland, Japan, and the USA. Our worldwide sales network is continuously expanding through commercial agreements with qualified distributors.
- VIA DELL’INNOVAZIONE, 9 - 20032 CORMANO (MI)
- INFO@ADLERORTHO.COM
- +39 02.6154371
- 02.615437222